|
11.4 Le-Chatelierís Principle
Once an equilibrium is established, no further change is apparent as long as the external conditions remain unchanged.
On changing the external conditions, whether the equilibrium will shift towards the reactants or the products can be predicted by the application of Le-Chatelierís principle which states that
When a stress (change in concentration, temperature or total pressure) is applied to a system at equilibrium, the system readjusts so as to relieve or offset the stress.
Application of Le-Chatelierís principle
In the synthesis of Ammonia (Haberís process)
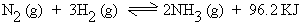
1 vol 3 vol 2 vol
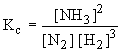
-
Effect of concentration : To increase
the yield of NH3, that is to favor the forward
reaction, concentration of N2 or preferably
H2 should be increased.
-
Effect of pressure : As there is decrease
in volume (from 4 to 2), the yield of NH3 increases
by increasing pressure.
-
Effect of temperature : The reaction
being exothermic, low temperature favors the reaction. However
at a low temperature, the rate of formation of NH3
is very slow, hence the reaction is carried out at optimum temperature
of 673 - 773 K.
**********
[next chapter]
|
Index
11.1 Types of Reactions
11.2 Equilibrium Law Expression
11.3 Factors Affecting Chemical Equilibrium
11.4 Le-Chatelier's Principle
Chapter 12
|