|
12.3 Amphoteric Substance
A substance which can act either as an acid or a base, depending on the nature of the other substance is called an amphoteric substance or amphiprotic.
Water can accept a proton and can act as a base. Similarly it can donate a proton and act as an acid.
HCl (g) + H2O 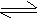 H3O+
+ Cl- H3O+
+ Cl-
Base
NH3
(g) + H2O 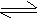 NH4+
+ OH- NH4+
+ OH-
Acid
[next page]
|