|
6.2 Boyle’s Law

Robert Boyle, in 1662, published a mathematical statement on the relation between the volume and pressure of gas at constant temperature called Boyle’s Law. This law states that "At constant temperature, volume of a definite mass of a dry gas is inversely proportional to its pressure."
It can be mathematically expressed as

The magnitude of constant depends on temperature, mass and nature of a gas.
Boyle’s law can be useful in calculating the volume of a gas at any required pressure if the volume at some other pressure is known by using the following equation.
P1V1 = P2V2 = K
This can be graphically represented as follows :
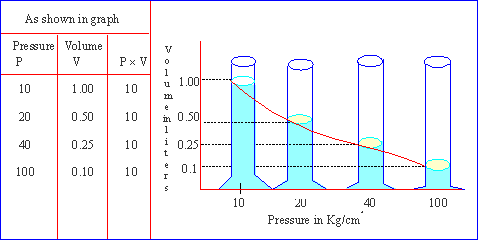 Click here to enlarge Click here to enlarge
|