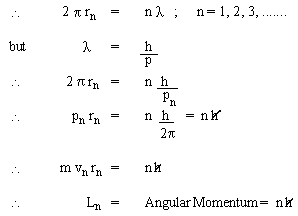33.4 Bohr's Atom
The 'permissible' orbits of electrons in atoms were like a commandment: thou shalt not ; in other words, there was no underlying principle to justify the quantization of angular momentum. However, de Broglie's matter-waves provides justification for permissible orbits. The matter-waves associated with electrons must form standing waves, which are formed in circular loops only when the waves join smoothly on themselves; i.e., the circumference must be equal to integral multiple of wave length.
i.e. Bohr's quantization rule for angular momentum of electrons in an atom.
|
33.1 Compton
scattering of photons Follow @Pinkmonkey_com  |