|
CHAPTER 34 : RADIOACTIVITY AND NUCLEAR STRUCTURE 34.1 Laws of radio - active transmutation The discovery of emissions of radiations from heavier elements like Uranium by Henry Bequerel in 1896, is known as radioactivity. Subsequent to this discovery extensive research carried out in this field established that there are three kinds of radiations so called because of their effect on photographic plates emitted by radioactive substances called a- rays, b- rays, g- rays. From experiments it was found that : (1) a- rays are beams of bare helium nuclei; with low penetration and high ionization power. They are deflected in electric and magnetic fields. They produce, fluorescence and phosphorescence. They are represented as 2He4.
(2) b- rays are beams of electrons, with moderate penetration and ionization power. They are deflected in electric and magnetic fields. They produce fluorescence and phosphorescence. They are represented as -1e0.
(3) g- rays are beams of Photons, with high penetration and low ionization power. They cannot be deflected in electric and magnetic field, can produce fluorescence and phosphorescence. They are represented as 0g0.
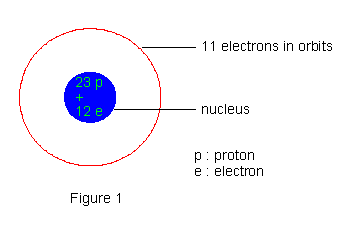
The origin of these radiations was traced to the nucleus from the observed fact that these radiations are much more energetic compared to the energy of electrons in atoms and compared to X- ray emissions from atoms; of course the possibility of an a- particle being ejected from an atom outside of its nucleus just does not arise. Hence, glimpses of nuclear structure were obtained. Thomson's discovery of positive rays or anal rays had lead to recognize protons as constituents of nucleus. In the initial stages electrons also were thought of as being present in the nucleus, primarily because of b- ray emission. Thus the structure of the sodium nucleus for example was thought of as made up of 23 protons and 12 electrons. With 11 electrons in orbits around the nucleus, the neutrality of atoms also could be achieved.
|
34.1 Laws of radio - active transmutation Follow @Pinkmonkey_com  |