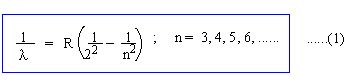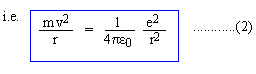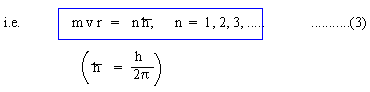CHAPTER 32 : BOHR'S THEORY OF HYDROGEN ATOM AND ITS SPECTRUM The development of Spectroscopy and gas discharge tubes enabled physicists in the second half of the 19th Century to analyze the spectrum of various gases, particularly that of Hydrogen gas. The analysis culminated in an empirical formula developed by Balmer (1885) to describe the visible line spectrum of the hydrogen atom.
where R is empirical constant called Rydberg's Constant (1.097 ´ 10 7m -1). The classical wave theory of light was again found to be completely inadequate while accounting for Balmer's formula. On the contrary the theory predicted continuous spectrum. Rutherford's nuclear atom model is also flawed on account of the classical theory because an electron in circular orbit would radiate away its mechanical energy due to its centripetal acceleration and would spiral into the nucleus destroying the stability of atom.
In 1913, Niels Bohr developed a remarkable theory based on extension of concepts of quantizations of Planck and Einstein. His theory is based on the following three postulates : (i) Rutherford's nuclear atom model is essentially correct.
(ii) Only those circular orbits of electrons are permissible in which the angular momentum of an electron is
These permissible orbits are 'steady' or 'stationary'.
(iii) Electrons in 'stationary' orbits do not radiate their energy ; only when electrons jump from a higher energy stationary orbit to a lower energy stationary orbit, the difference of energy (DE) is radiated off in the form of a photon of light of frequency ( n ) given by Einstein's relation.
Is it not strange that an electron obeys Newton's law of motion Equation (2)? Yet the consequence of this motion namely the radiation of energy of motion is prohibited; not only that, but the mechanism of radiation emitted is totally different from classical mechanism. To some extent the concept of photon seems to provide some justification; but that is about all. The only logic, that apparently justifies these assumptions is that they work. |
32 : Bohr's theory of hydrogen
atom and its spectrum Follow @Pinkmonkey_com  |