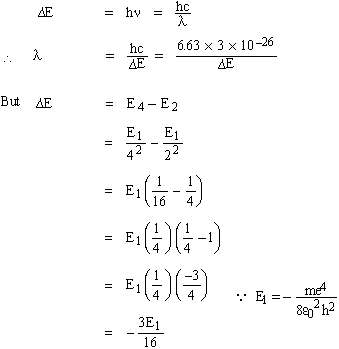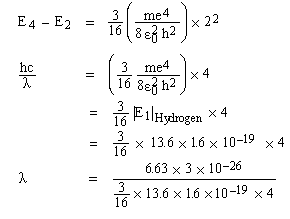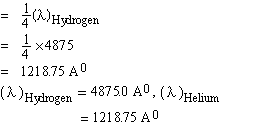Solved Problem 1. If an electron in an hydrogen atom jumps from the 4th to the 2nd permissible orbit, what will be the wave length of light emitted? The ground state energy of hydrogen atom is -13.6 eV. If the same transition occurs in singly ionized helium atom then what would be the wave length?
Solution
*********** |
Chapter 32 : Bohr's theory of
hydrogen atom and its spectrum Follow @Pinkmonkey_com  |