|
PinkMonkey Online Study Guide-Biology
2) Disaccharides: These carbohydrates contain two monosaccharides linked together and accordingly they are known as:
(a) Disaccharide : contains two monosaccharides e.g. lactose, maltose, sucrose
Maltose ® Glucose +
Glucose
Sucrose ® Glucose + Fructose
Lactose ® Glucose + Galactose
(b) trisaccharide: containing 3 monosaccharides. e.g. raffinose
(c) tetrasaccharide: containing 4 monosaccharide e.g stachyose
3) Polysaccharides
General formula n (C6H10O5).
These complex carbohydrates are formed by chains of at least ten
monosaccharides.
They are of two types:
(a) Homoglycans: containing only one type
of monosaccharide (e.g. glycogen, starch, cellulose,
contain only glucose molecules). Starch is a very important
polysaccharide because it is formed through a chain
of hundreds or thousands of glucose units. Carbohydrates in plants
are stored in the form of starches. Starch contained
in energy rich food like rice, corn, and potatoes form part of the
staple diet of most people.
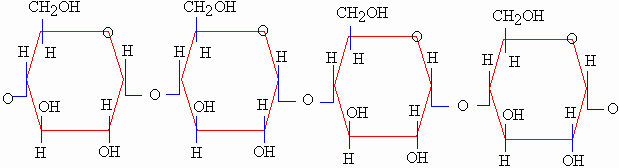
Starch
Click here to enlarge
A second important polysaccharide is glycogen. Glycogen
also contains thousands of glucose chains; the difference from starch
though is in its branching pattern. Glucose is stored in the human
liver in the form of glycogen.
Another important polysaccharide is cellulose.
Cellulose is used primarily as a structural carbohydrate. It is also composed
of glucose units, linked in a different orientation but the units cannot
be released from one another except by a few species of organisms. Wood
is formed from cellulose. Even the cell wall of all plants is made up
of cellulose. Cotton and paper are also cellulose products.
(b) Heteroglucans: contain more than
one type of monosaccharide linked together (e.g. mucilage, gum etc.)
4) Proteins and its derivatives
Proteins are the fundamental chemical compounds
of the protoplasm indispensable for vital life processes. They are
complex, large molecules each containing thousands of atoms. proteins
contain nitrogen in addition to carbon, hydrogen and oxygen; they
usually also contain phosphorus and sulfur. These compounds are
polymers of unit structures called amino acids, represented chemically
as:
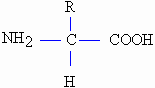
amino acid
-NH2 is an amino group, - COOH is the carboxyl
group, ,and R represents the variable chain forming different amino acids.
There are 20 diferent of amino acids. The amino acids differ depending
on the nature of the R group. Examples of. amino acids are valine, alanine,
glutamic acid, tyrosine and histidine.
Two molecules of amino acids are joined by the
carboxyl group of one ammino acid with the amino group of
the other by loss of one molecule of water. This process is called
dehydration synthesis and the bond thus formed between
two molecules is referred to as the peptide or peptide bond.
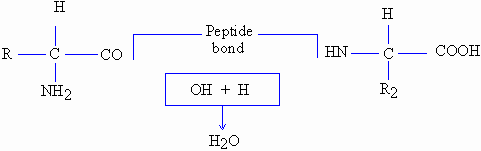
Formation of dipeptide
Click here to enlarge
There are 3 types of proteins namely :
(1) Simple proteins: like albumins and globulins
formed by group of amino acids only.
(2) Derived proteins: like proteose and
peptones which are hydrolytic cleavage products of complex proteins.
(3) Conjugated proteins: like nucleo proteins
(Proteins + nucleic acid), lipoproteins (protein + lipid), or glycoproteins
(protein + carbohydrates) which are formed by the combination of
proteins with some non-protein molecule. This non-protein portion
is called Prosthetic group.
All living things require protein for survival. In fact
an organism is constructed by means of proteins. All living things
then, in any form - liquid, solid, or plasma - contain proteins. Protein
is also seen as a supporting tissue with main tissue. Bone, tendons, muscle,
cartilage, ligaments are all formed of protein.
Enzymes are a specified class of proteins. Enzymes act as catalysts in
chemical reactions of the body. They are not used up by the reaction,
rather they remain chemically unchanged and available to catalyze succeeding
reactions.
|