27.2 Theory of Diamagnetism and Paramagnetism
The origin of the magnetic behavior of any substance can be traced to the atomic structure and
arrangements of a large number of atoms in bulk matter.
Every atom can be considered to be a short magnet having essentially dipole moment on account
of similarity of orbiting electrons with a current carrying coil.
However, electrons have two types of angular momenta and each type of angular momentum is
associated with magnetic dipole moment. These angular momenta of electrons are called orbital angular momentum
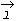 and spin angular momentu and spin angular momentu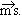 . Rigorous treatment is out of the scope, but it should be mentioned that . Rigorous treatment is out of the scope, but it should be mentioned that
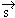 is purely a Quantum degree of freedom. The resultant dipole moments due to is purely a Quantum degree of freedom. The resultant dipole moments due to
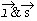 & s and the effect of the external fields on them, result into three types of magnetism. & s and the effect of the external fields on them, result into three types of magnetism.
The dipole moment 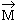 s,
due to spin angular momentum s,
due to spin angular momentum 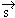 of each electron is not appreciably affected by moderate fields,
whereas
of each electron is not appreciably affected by moderate fields,
whereas 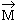 l
due to l
due to 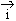 (which is in opposite direction i.e.
(which is in opposite direction i.e. 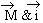 &
i are anti-parallel to each other) is found to have changed, the
change being always opposite in direction to the applied magnetic
field. This qualitative description enables us to understand the
magnetic behavior as follows. &
i are anti-parallel to each other) is found to have changed, the
change being always opposite in direction to the applied magnetic
field. This qualitative description enables us to understand the
magnetic behavior as follows.
Diamagnets
Individual atoms have total 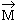 s = 0 due to all (even numbered) electrons. According to Pauli's
exclusion principle; total s = 0 due to all (even numbered) electrons. According to Pauli's
exclusion principle; total 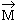 l
# 0. But due to randomness in arrangement of a large number of atoms, the
substance has net l
# 0. But due to randomness in arrangement of a large number of atoms, the
substance has net 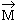 = 0. However, when placed in a uniform external field, the atoms will orient their changed = 0. However, when placed in a uniform external field, the atoms will orient their changed
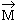 l anti-parallel to the field. Hence, net l anti-parallel to the field. Hence, net 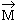 induced in the substance will also be anti-parallel to the field B
and such a substance will be repelled away. induced in the substance will also be anti-parallel to the field B
and such a substance will be repelled away.
Paramagnetism
Individual atoms have total
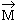 l # 0 due to all (odd numbered) electrons. Each atom is itself a short
magnet, having net dipole moment l # 0 due to all (odd numbered) electrons. Each atom is itself a short
magnet, having net dipole moment 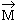 # 0. However, randomness (due to thermal agitation) of the orientations of # 0. However, randomness (due to thermal agitation) of the orientations of
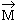 of all atoms implies that total of all atoms implies that total 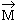 = 0 in the substance. But, when external field is applied, the alignment
of net = 0 in the substance. But, when external field is applied, the alignment
of net 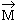 of each atom becomes parallel to of each atom becomes parallel to 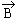 . Despite the effect on . Despite the effect on
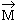 l being opposite to that on l being opposite to that on 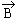 , there
is no effect of , there
is no effect of 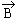 on on 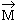 ( which is greater than ( which is greater than
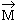 l ). This results in a resultant l ). This results in a resultant 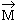 in in
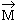 s direction and this is aligned along s direction and this is aligned along 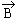 by the torque exerted by by the torque exerted by 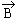 . .
The substance therefore acquires small net dipole moment that is induced, parallel to the field, and
therefore weak attraction occurs. The induced dipole moment reduces to zero, i.e. the randomness in orientations of
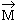 s of each atoms reverts when the external field in removed. The substance therefore becomes de-magnetized. The
paramagnets are therefore temporary magnets, having magnetism induced only as long as external fields
are imposed. s of each atoms reverts when the external field in removed. The substance therefore becomes de-magnetized. The
paramagnets are therefore temporary magnets, having magnetism induced only as long as external fields
are imposed.
[next page]
|

