The ETS reactions are oxidation-reduction (Redox)
reactions. During the transfer of hydrogen/electrons through the
chain, each carrier molecule is alternately reduced and oxidized.
For example, when a carrier accepts hydrogen/electron it gets reduced.
On the other hand, the reduced carrier is oxidized when it transfers
the hydrogen/electron to the next carrier in the chain.
There is a net flow of electrons through the ETS
from more electronegative redox potential (i.e. from NADH2
or FADH2) to the more electropositive redox potential
(i.e. to oxygen).
Click Here To Enlarge
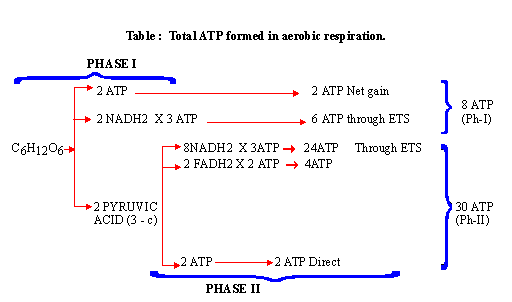
Overall result of aerobic respiration
Complete oxidation of one molecule of 6-C glucose
through aerobic respiration (involving glycolysis, Kreb’s cycle,
ETS, etc.) results in —
(a) release of 6#CO2
(b) Utilization of 6#O2 and
(c) Formation of 6#H2O.
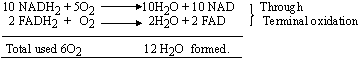
However, 6H2O are used up in Kreb’s
cycle (involving 2 pyruvic acid molecules).
\ 12 H2O formed - 6H2O
used = 6H2O net gain