|
CHAPTER 13 : ORGANIC CHEMISTRY
13.1 Introduction
A wide range of compounds existing either naturally or
synthetically prepared are known to possess the element carbon. This forms
a branch of chemistry, known as Organic Chemistry. Over three million
different organic compounds have been characterized & every year tens
of thousands of new substances are added to the list.

Antoine Lavoisier
In 1774, Antoine Lavoisier
showed that compounds obtained from vegetable and animal sources always contained at least carbon and
hydrogen.
Carbon is not the only element found in organic compounds. Hydrogen
atoms are almost always present. In addition organic compounds often
contain atoms of oxygen, nitrogen, phosphorus, sulfur or the halogens.
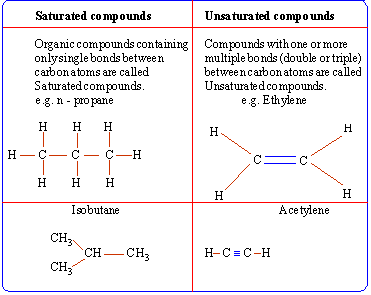
The bonds formed by carbon with other carbon atoms or other atoms
can be single, double or triple bonds.
Standard conventions used to represent these bonds
are as follows:
|
No. of lines
|
Type of bond
|
Example
|
|
Single
|
Single
|
C - C, C -
H
|
|
Double
|
double
|
C = C, C = O
|
|
Triple
|
triple
|
C º C, C º
N
|
Organic compounds differ considerably from
inorganic compounds. The following comparison illustrates the difference
between organic and inorganic compounds. Remember that there are
exceptions to every point of comparison.
| |
Organic compounds
|
Inorganic compounds
|
|
(i)
|
Relatively few elements (mainly C, H, O, N, S, P, F, Cl, Br, I)
are involved.
|
All elements are involved.
|
|
(ii)
|
Bonds are covalent.
|
Bonds are ionic or electrovalent.
|
|
(iii)
|
Sparingly soluble or insoluble in water but soluble in non-aqueous solvents (organic
solvents).
|
Soluble in water but insoluble in organic solvents.
|
|
(iv)
|
Volatile in nature.
|
Non-volatile in nature.
|
|
(v)
|
Non electrolytes
|
Electrolytes
|
|
(vi)
|
Rate of reaction is slow and a catalyst is needed.
|
Rate of reaction is fast and a catalyst is not
needed.
|
|
(vii)
|
Mostly inflammable
|
Not inflammable
|
|
(viii)
|
Complex structure
|
Simple structure
|
|
(ix)
|
Isomerism is very common.
|
Isomerism is not very common.
|
|
Index
13.1
Introduction
13.2 Functional Groups
13.3
Formula of Organic Compounds
13.4 Geometrical Isomerism
13.5 Hydrocarbons
13.6 Homologous Compounds
Chapter 1
|