|
CHAPTER 10 : ELEMENTS OF CHEMICAL THERMODYNAMICS
10.1 Introduction
Thermodynamics is the study of energy and
energy changes. Energy is the capacity to work. It is present
in various forms like potential energy, kinetic energy, light energy,
electrical energy etc.
Energy and energy changes are always calculated in terms of heat energy as there is a natural tendency that all other forms of energy get easily and finally converted into heat energy.
The branch of thermodynamics dealing with energy changes during chemical transformation
is called chemical thermodynamics.
Types of thermodynamic systems
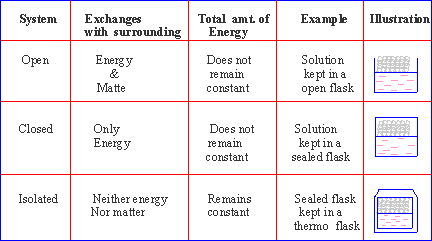 Click here to enlarge
Click here to enlarge
Homogenous system : A system is homogenous
when it has some chemical composition throughout. e.g. mixture of
gases or true solution of solid in liquid.
Heterogenous system : Two or more different
phases which are homogenous but separated by a boundary. e.g. Ice
in water.
|