|
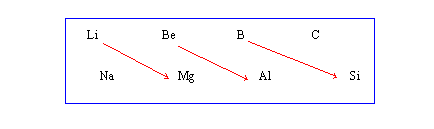
Diagonal Relationships :
On moving diagonally across the periodic table, the elements show certain similarities in their properties which are quite prominent in some cases as shown below. This is called a diagonal relationship.
Classification of elements on the basis of electronic configuration:
From the view point of electronic configuration the elements can be subdivided into four major sub groups (Figure 1). They can be classified as s,p,d and f block elements.
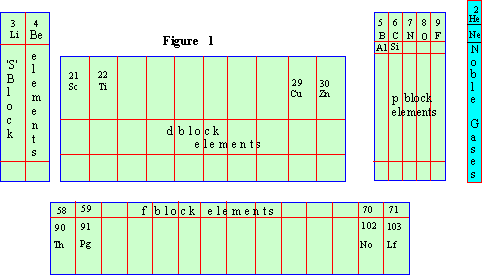
1. s-block elements : These have two atoms
and include groups I A & II A. They contain one or two electrons
in their outer S-orbital. Strongly electropositive metals.
The group I A elements are alkali metals. Elements with two
electrons in the outer orbit are called alkaline earth metals.
2. p-block elements : The elements from
groups III A to VII A are included in this type. These are generally
non-metals. These elements are generally non-conductors
with the exception being Silicon and Germanium
which are semi-conductors.
3. d-block elements : These are the elements
in which the last electron is placed in d-orbital of penultimate
energy level. These elements are also known as transitional elements.
All are metallic and have high melting and boiling points.
Good conductors of heat and energy.
4. f-block elements : The transition elements
in which pre-penultimate f-sub orbital is being filled up are called
as inner-transition elements. There are two series of these
elements :
1) Lanthanides & 2) Actinides. Their compounds are colored and are usually paramagnetic.
5. zero-group elements : Earlier these elements
were known as inert or Noble gases. They are chemically unreactive.
They are incapable of forming any chemical compounds.
|