|
CHAPTER 5 : STATES OF MATTER
5.1 The Physical Status of Matter
There are mainly three different kinds of the physical
states of matter, namely:
- Solid
- Liquid
- Gas
Plasma a fourth class of matter has also been identified.
These states of matter are also termed as phases.
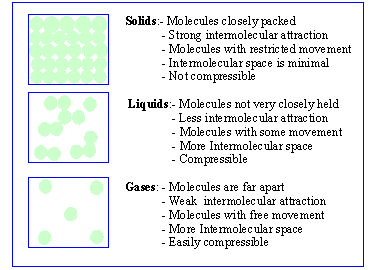
1) Solid : Solids are characterized by their definite
shape and also their considerable mechanical strength and rigidity. Solids
tend to resist the deformation of their shape due to strong intra molecular
forces and absence of the translatory motion of the structural units (atoms,
ions etc). A solid is relatively non compressible, i.e. temperature and
pressure have only a slight effect on its volume.
Solids are broadly classified as crystalline or amorphous.
Crystalline solids : Here the atoms are arranged
in a definite pattern which is constantly repeated.
Amorphous solids : These have no definite geometrical
form.
2) Liquid : A liquid has no definite shape and
it takes the shape of the vessel containing it. Like solids, the volume
of a liquid is slightly altered by variations in temperature and pressure.
Liquids have three typical physical properties, namely:
i) Vapor pressure : A Liquid when kept in a closed
container vaporizes into the free space above it. The process of vaporization
will continue till the equilibrium is reached between liquid and vapor.
The pressure at which the liquid and vapor can co-exist is called the
vapor pressure of the liquid at a given temperature.
ii) Surface tension : The surface of a liquid
is always in a state of tension because a molecule at the surface is attracted
towards the bulk by a force much greater than that drawing it toward the
vapor where the attracting molecules are more widely spread. Due to this,
a certain force is required to penetrate along any line in the surface.
This force is called surface tension.
iii) Viscosity : It determines the flow of the
liquid. It is the internal friction between layers of the liquid. Higher
the rate of friction, greater the viscosity of the liquid and its flow
will be retarded. Conversely, a lower rate of friction lessens the viscosity
and makes the liquid more fluid.
3) Gases : A gas has no bounding surface at all and
will occupy completely any vessel in which it is filled. It has no
definite volume or shape and can be easily expanded or compressed.
Laws governing behavior of gases will be dealt with in
detail in the next chapter.
Water is the ideal example to show the different states
of matter.
Water when cooled to 00C becomes solid. When
the temperature of solid water is raised it becomes liquid. If the liquid
is heated to 1000C it gets converted to steam or vapor (The
Gas phase).
Almost all chemical substances can exist in more than
one physical state (phase) depending on external pressure and temperature.
The following table illustrates different states of matter
and their physical properties.
Table 9
|
|
Property
|
States of matter
|
|
|
|
Solid
|
Liquid
|
Gas(Vapor)
|
|
i)
|
Shape
|
definite
|
indefinite
|
indefinite
|
|
ii)
|
Volume
|
definite
|
definite
|
indefinite
|
|
iii)
|
Molecular
Bonding
|
very strong
|
strong
|
Weak
|
|
iv)
|
Examples
|
NaCl
ZnSO4
|
H2O
Petrol
|
H2 , CO2
LPG
|
|
|
|
The Molecular mode of Solids, Liquids, Gases
Plasma : This is the fourth state of matter. It is a type of gas
containing positively and negatively charged particles in approximately
equal numbers and present in the sun and most stars.
|