|
The CO2 system : The Phase diagram here is
very similar to that of water as shown in figure 19.
Figure 19
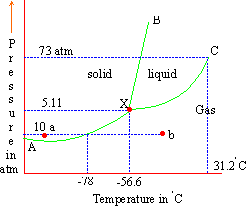
In the above figure,
XA is the vapor pressure curve of solid CO2
XB is the freezing point curve of liquid CO2
XC is the vapor pressure curve of liquid/gas CO2
Point C at 73 atm and 31.20C is the critical point where it is difficult to distinguish between liquid and gas. Point X is the triple point at 5.11 atm and -56.60C.
The graph reveals that liquid CO2 cannot exist
below 5 atm.
Suppose we have solid CO2 at a very low temperature at point ‘a’. If at the same pressure, the temperature is increased gradually, the solid will change from ‘a’ to ‘b’ where it would become gas without melting, i.e. without passing through the liquid state. This is called sublimation.
Similarly, in the case of Napthalene, Iodine and Camphor etc.
the vapor pressure is appreciable and rises with increasing temperature.
Vapor pressure reaches atmospheric pressure before it reaches its melting
point. As a result it vaporizes and sublimation occurs.
|