31.2 Rutherford's Experiments on the scattering of the a- particle and the Nuclear atom model
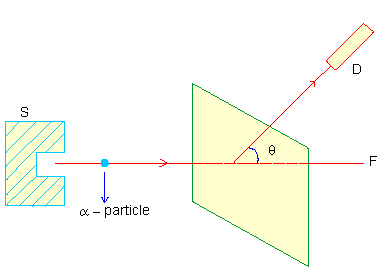
Figure 2 The schematic arrangement of the experiment is as shown in Figure 2.
A collimated beam of a-particles (Base Helium nuclei, having 2 units of positive charge and 4 units of mass) from a radioactive source is made to be ncident on a thin foil of gold. The number of a- particles at various scattering angles q are detected by the detector (Geiger-Muller tube for example) D. The results of the experiments were :
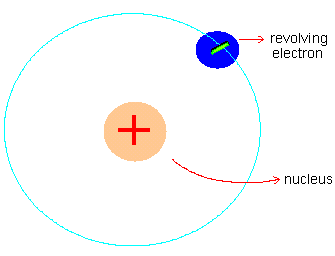
These results were totally unexpected, as the Thomson model of the atom will hardly allow the atoms to produce an intense electric field necessary to repel incoming a- particles (even stop them) and hence scatter them by large angles (retrace their incident path). In Rutherford's own words, "It was like a shell fired at tissue paper rebounced and hit you." Rutherford discarded Thomson's atom model and instead proposed the currently accepted nuclear atom model. The positive charge and also almost the whole mass of the atom is concentrated in a tiny volume (10-4 times smaller in size than atoms) at the center of the atom called the nucleus; the electrons revolve around the nucleus in circular orbits. (See figure 3)
Figure 3
|
31.1 Thomson's
Jelly and Plum-Pudding Model Follow @Pinkmonkey_com  |