|
Illustration
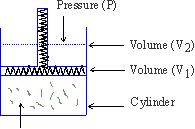
\ W = P ´ D V ( D V = V2 - V1 )
Example : CO2 is expanded by supplying 2.343 KJ of heat. If the work done in expansion is 1.4644 KJ calculate the energy change of CO2.
q = + 2.343 KJ W = 1.4644 KJ
\ DE = 2.343 - 1.4644 = 0.8786 KJ
\ Internal energy change, DE
= 0.8786 KJ
Example : A gas expanding against a constant pressure of 202.65 KPa from 10 to 20 dm3, absorbs 1255.2 J of heat. Calculate the change in the internal energy of the gas.
DE = q - W
DE = q - P( V2 - V1 )
= 1255.2 - 202.65 ( 10 - 20 )
= - 3281.7 J.
|