|
Problems
1. If Kc for the equation,
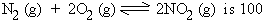
find out KcI and KcII for
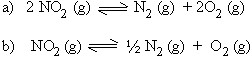
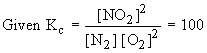
Solution :
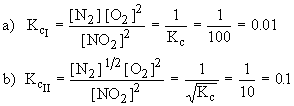
2) When 0.4 mole of PCl5 is heated to 523 K in a 10 dm3 container, an equilibrium is established in which 0.25 mole Cl2 is present.
Find out Kc for the dissociation of PCl5 at 523 K.
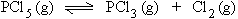
Initially 0.4 mole 0 mole 0 mole
At equili. (0.4 - 0.25) 0.25 mole 0.25 mole
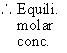 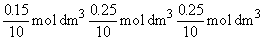
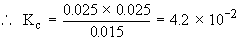
3) The equilibrium constant KP for the dissociation
of PCl5 at 523 K and 101.3 KPa pressure is
180.3. Calculate its percent dissociation at 523 K.
Initially 1 mole 0 mole 0 mole
At equilibrium (1 - x) mole x mole x mole
(Total number of moles at equilibrium = 1- x + x + x = 1+ x )
If P is total pressure then partial pressure at equilibrium
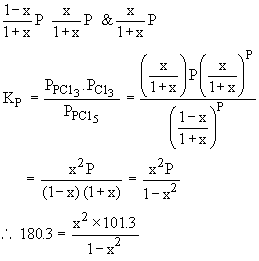
180.3 - 180.3x2 = x2 ´ 101.3
101.3x2 + 180.3x2 = 180.3
\ x2 =
0.64 \ x = 0.8
Percent dissociation = 0.8 ´ 100 = 80%
|