|
In 1923, J. N. Bronsted and T. M. Lowry proposed definitions
of acids and bases in aqueous as well as non aqueous solutions according
to which
An acid is defined as a substance having a tendency to lose or to donate one or more protons and,
A base is defined as a substance having tendency to accept or add
a proton.
Example :
NH3
(g) + HCl (g) 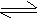 NH4+
+ Cl- NH4+
+ Cl-
Base Acid
Here HCl is proton donor (hence acid) and ammonia is a proton acceptor (hence base)
Other examples are :
1) NH3 (aq) + H2O
(l) 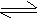 NH4+
(aq) + OH- (aq) NH4+
(aq) + OH- (aq)
Base Acid
2) NH3 (aq) + H3O+
(aq) 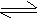 NH4+
(aq) + H2O (l) NH4+
(aq) + H2O (l)
Base Acid
[next page]
|