|
6.3 Charle's Law

Jacques Charles, in 1787, formulated the relationship between the volume and temperature of a given mass of a dry gas at constant pressure called Charles' Law which states that "At constant pressure, the volume of a fixed mass of dry gas is directly proportional to the absolute temperature;
It can be mathematically expressed as
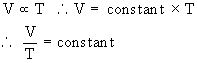
The magnitude of constant depends on pressure, mass and nature of a gas.
Charles' law is useful for calculating the volume of a gas at any required temperature if the volume at some other temperature is known by using the following equation.
V1 = T1
V2 = T2
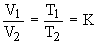
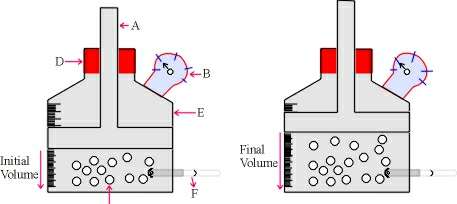
Illustration of Charles' Law:
 Click here to enlarge
Click here to enlarge
A : Piston
B : Pressure gauge
C : Gas Molecule
D : Rubber cork
E : Vessel
F : Thermometer
Example :
A gas at constant pressure is kept at 1000C. On decreasing the temperature to 500C, the gas occupies a volume of 800 ml. Find the initial volume of the gas.
V1 = ? T1 = 1000C = 100 + 273 K = 373 K
V2 = 800 ml T2 = 500C = 50 + 273 K = 323 K
According to Charles' Law
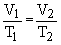
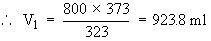
Initial volume of the gas was 923.8 ml.
|