|
6.4 Pressure Temperature Law
The Pressure and Temperature law is similar to Charles' law. It states that "For a given mass of a dry gas, the pressure is directly proportional to the absolute temperature, if the volume is kept constant."
It can be mathematically expressed as
P µ T, if V is constant
P = constant ´ T
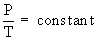
This law can be useful in calculating the pressure of a gas at any required temperature if the pressure at some other temperature is known by using the following equation :
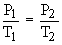
If 25 dm3 of a gas at 36.5 KPa is cooled from 298 K to 136 K, keeping the volume constant, the final pressure can be calculated as follows :
P1 = 36.5 KPa / P2 = ?
T1 = 298 K T2 = 136 K
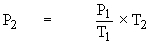
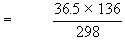
= 16.66 KPa
|