|
6.5 Gay Lussac Law

In 1805, Gay Lussac observed a phenomenon based on his experiments
on different gases. He framed his observations in the Law of combining
volumes of Gases which states that "when gases react they do so
in volumes which bear simple whole number ratios to one another
and to the volume of the products, if gaseous, when measured at
the same conditions of pressure and temperature.
Example
1. One volume of H2 combines with one volume of Br2
to form two volumes of HBr gas
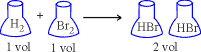
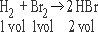
Ratio of reactions and products 1 : 1 : 2
2. One volume of N2 combines with 3 volumes of H2 to produce two volumes of NH3 gas
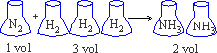
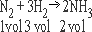
Ratio of reactions and products 1 : 3 : 2
Example
500 cm3 of Nitric oxide (NO) reacted with 300 cm3 of O2 to form nitrogen dioxide (NO2). What would be the composition of final mass?
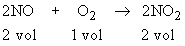
\ 2 vol of NO requires 1 vol O2
2 cm3 of NO requires 1 cm3 O2
\ 500 cm3 will require 250 cm3
of O2
Now
2 vol of NO yields 2 vol of NO2
\ 2 cm3 of NO yields 2 cm3
of NO2
\ 500 cm3 NO yields 500 cm3
of NO2
The resulting mixture consists of
1. Unused O2 = (300 - 250) = 50cm3
2. Product NO2 formed = 500 cm3
|