|
b) To find out the relationship between the molecular weight
of a gaseous substance and its vapor density:
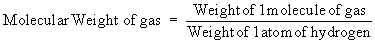
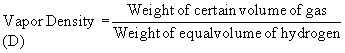
According to Avogadro’s Law the above equation
can be written as
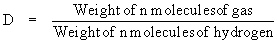
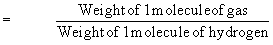
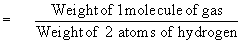
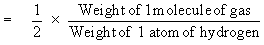 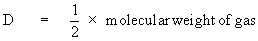
or Molecular weight = 2 ´ vapor density
c) Gram-molecular volume of a gas:
Vapor density (D) of a gas is expressed as
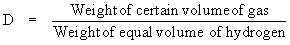
\ For 1 dm3 volume at
N.T.P. condition
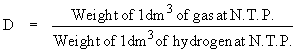

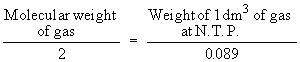
\ The molecular weight in grams of any gas occupies 22.4 dm3
at N.T.P.
This is called Gram-Molecular volume.
|