Example :
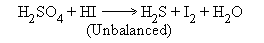
Step I: 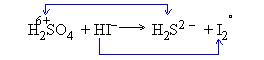
Step II : 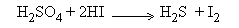
Step III : 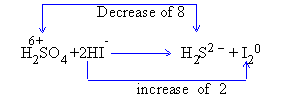
Step IV : 
Step V : 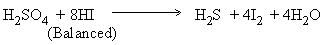
Oxidising and Reducing agents
Oxidising agent : It is a substance which has the tendency to
gain one or more electrons from another substance.
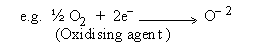
As a result the oxidising agent itself undergoes reduction during redox
reaction.
Reducing agent : It is a substance which has the tendency to
lose one or more electrons to another substance.
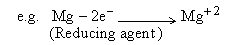
As a result the reducing agent itself undergoes oxidation during redox
reaction.
| Some common oxidising
agents |
Common reducing agents |
| Oxygen |
Hydrogen |
| Chlorine |
Carbon |
| Conc.H2SO4 |
Carbon monoxide |
| Conc. dil.
HNO3 |
Ammonia |
Oxides like:
H2O2, MnO2,
PbO2, K2Cr2O2 |
H2S
SO2 |
**********
[next chapter]
|
Index
7.1
Introduction
7.2
Oxidation Numbers
7.3
Balancing Reactions
Chapter 8
|