|
7.3 Balancing Redox Reactions
A chemical reaction is treated as a balanced one when the number of atoms of each element is equal in the reactants and products.
How to balance redox reactions
1) Write the unbalanced equation and identify the elements which undergo change in oxidation number.
For convenience draw a line between them
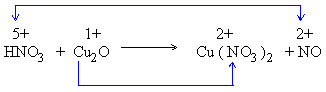
2) Balance the number of atoms of each element undergoing change in oxidation number
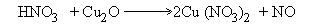
3) Indicate total change in oxidation number
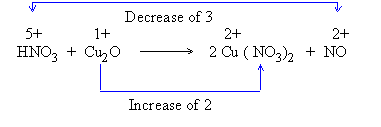
4) Multiply the total charges as given below to equal the gain and loss of electrons
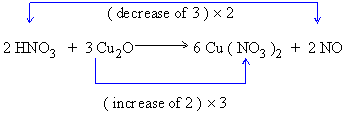
5) Balance the remainder of atoms by inspection, if necessary add acid or base and water as needed.
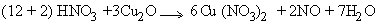
The balanced equation is :-
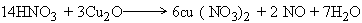
|