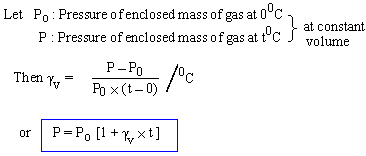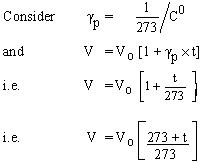|
12.3 Gases
Gases neither have definite size (volume) nor definite shape. Moreover, the changes in pressure are equally important (in case of solids & liquids these changes are negligible) as the changes in volume, when gases are heated. Therefore to determine the unique relation between changes in volume and temperature, the pressure must be kept constant and to determine unique relation between changes in pressure and temperature the volume must be kept constant. Hence the following two coefficients are defined for enclosed masses of gases.
Coefficient of Volume expansion at constant pressure : gp
The change in volume, above the volume at 00 C per unit original volume at 0 0 C per 10 C change in volume, at constant pressure, is called gp . 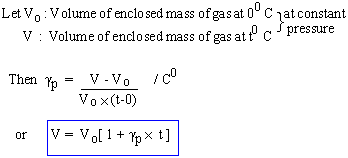
Note: Experimentally it is found that
Coefficient of Pressure expansion at constant Volume:g v
The changes in pressure, above the pressure at 00C per unit original pressure at 00C per 10C change in temperature, at constant volume, is called gv .
Note: Experimentally it is found that
Kelvin or Absolute or Ideal Gas Scale of Temperature
Therefore at t = -2730C the volume of gas becomes V = 0, which must be treated as the limit. Thus, if a gas were to always remain a gas (therefore called ideal gas) without getting liquefied then the lowest possible temperature to which it can be cooled down must be - 2730C and not less than that (for the volume then will become negative; clearly absurd proposal). Although, the Celsius Scale of temperature is an objective measure, it is still dependent on properties of one particular substance viz. water; whereas all ideals gases tend to attain V = 0 at t = -2730C. Therefore, it is better to redefine the temperature scale in terms of behavior of all the ideal gases, irrespective of their chemical nature, rather than in terms of one particular substance viz. water.
The new temperature scale was introduced by Lord Kelvin, hence it is called the Kelvin Scale
of temperature and it is defined as It is obvious that just as - 2730C is the lowest possible temperature that can occur in nature, 00K is also the lowest possible temperature; hence 00K is the absolute zero of temperature. Since Kelvin Scale of temperature is based on the behaviour of Ideal gases, it is also known as the Ideal gas scale of temperatures. In Chapter 15 it will be shown that a scale of temperature can be defined independent of any substance; this scale of temperature can be called Absolute Scale and it will be shown that it is uniquely related to Kelvin Scale, hence Kelvin Scale is called also as Absolute Scale of temperatures.
|
12.1 Solids
Follow @Pinkmonkey_com  |