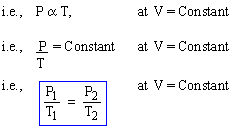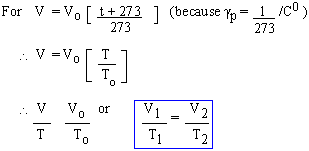|
12.4 Gas Laws
The behavior of enclosed masses of ideal gases is determined by the relations between P1 V or P1T, or V1T when the third quantity T or V or P respectively, is maintained constant; these relations were obtained experimentally by Boyle, Gay-Lussac, Charles respectively.
Boyle's Law
The Pressure (P) of an ideal gas of enclosed mass varies inversely with its Volume (V) if temperature (T) is kept constant. 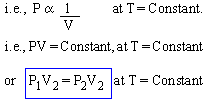
Gay-Lussac's Law
The pressure (P) of enclosed mass of an ideal gas varies directly with its temperature (T) if volume (V) is kept constant.
Note: This law is obvious from the definition of gv. 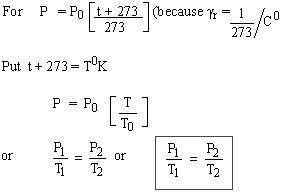
Charles' Law
The volume (V) of an enclosed mass of ideal gas varies directly with its temperature (T) if pressure (P) is kept constant. 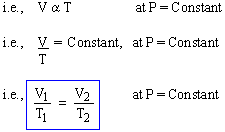
Note: This law is obvious from definition of gp.
|
12.1 Solids
Follow @Pinkmonkey_com  |