|
Note
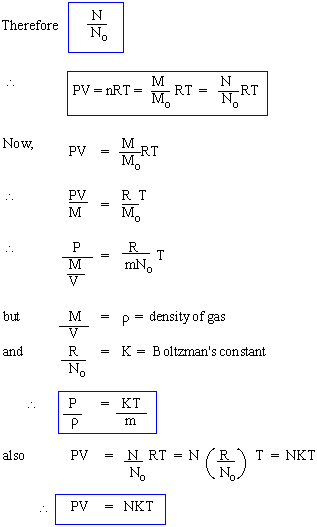
It is founded that the value of constant is dependent on the enclosed mass of gas for a given gas and is also found to differ for different gases of 1 unit of mass. However, it is found that if 1 mol. mass ( Mass numerically equivalent in gm to molecular weight, e.g., 2 gm for H 2, 32 gm for O2 , 28 gm for N2 , etc.) of any ideal gas is chosen then the value of the constant is same for all gases. This universal constant (same for all gases) is denoted usually by 'R' and is called the universal gas constant.
The ideal gas equation therefore is normally written as
The no. of molecules is defined as, the ratio of actual mass of gas M to its molecular weight (Mo) 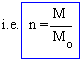
If m is the mass of each molecule of a given gas and N is the no.of molecules that make up the
mass = M; whereas No= Avogadro's no. = Number of molecules in 1 mole of the gas (any gas), then M
= mN & Mo = mNo.
|
12.1 Solids
Follow @Pinkmonkey_com  |