|
11.2 Equilibrium Law Expression
Let us consider a reversible homogenous reaction.
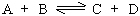
According to the law of mass action, the rate of forward reaction ( rf) is
rf = Kf
[ A ] [ B ]
and that of the backward reaction is
rb = Kb [
C ] [ D ]
Kb and Kf are the rate constants.
The rate of the net reaction is :
rn = rf - rb
At equilibrium, there is no net reaction i.e.
rf - rb = 0 \ rf
= rb
\ Kf [ A ] [ B ] = Kb
[ C ] [ D ]
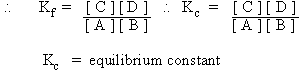
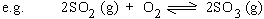
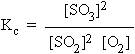
The equilibrium constant expressed in terms of partial pressures (Kp).
Equilibrium constants for gaseous reaction can be based on the partial pressure of a gas as the partial pressure of a gas is directly proportional to its molar concentration for a given temperature.
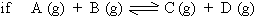
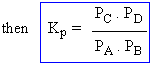
|