|
CHAPTER 12 : ACIDS, BASES AND SALTS
12.1 Lowry and Bronsted Concepts of Acids and Bases
The classification of substances of acids was at first suggested
by their sour taste (Latin acidus = Sour) and alkali (Arabic
alkali = ashes of a plant) were taken as those substances
that could reverse or neutralize the action of acids.
The classical definition of
ACID : as a substance whose water solution
i) turns blue litmus red
ii) neutralizes base
iii) reacts with active metals like Zn, Mg, Cu etc with
evolution of H2
iv) having sour taste
v) decomposes carbonates
The following chart shows different types of acid.
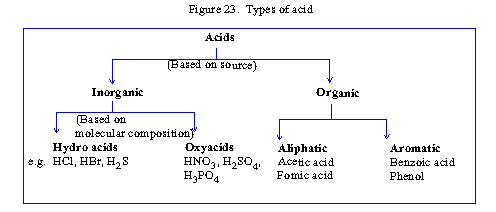
Click here to
Enlarge
Figure 23.
BASE : as a substance whose water solution
i) turns red litmus blue
ii) neutralizes acid to produce salts
iii) tastes bitter
iv) soapy to touch
v) reacts with few metals like Zn, Al, Pb etc liberating
hydrogen.
Examples : NaOH, KOH, NH4OH
[next
page]
|