|
12.9 Polyprotic Acids
Polyprotic acids can be defined as those acids that contain more
than one acid hydrogen (proton) per molecule.
e.g. H2SO4,
Oxalic acid (H2C2O4),
H3PO4,
arsenic acid (H3HSO4)
These acids ionize in a step wise manner (number of steps depends on number of acid hydrogen) and there is an ionization constant for each step.
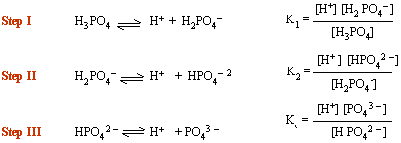
In all polyprotic acids
K1
> K2
> K3
i.e. primary ionization is stronger than secondary which is
in turn stronger than tertiary.
The ionization constant of some polyprotic acids are given in the following table:
[next page]
|