|
A positive ion is always smaller than the neutral atom ( Na is 1.570A
and Na+ is 0.950A
) and a negative ion is always bigger than the neutral atom ( F
is 0.720A and F- is 1.360A
)
Within a period ( as shown in the Table ) isoelectronic positive
ions ( having same number of electrons ) show a decrease in ionic
radium from left to right due to increase in nuclear charge ( Na+
> Mg2+
> Al3+
). Similarly for isoelectronic negative ions, ionic radium decreases
from left to right ( O2
- > F- ).
Within a group of periodic table, similarly charged ions increase in size from top to bottom
( Li+ < Na+
< K+ or F - < Cl-
< Br- < I - ) due to more electron shells.
The above table can be used to compare the strengths of ionic bonds in two compound in same medium.
Example : According to Coulomb’s law
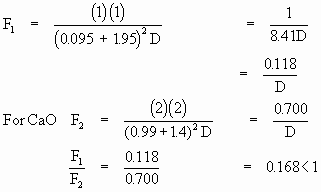
The strength of the CaO bond is more than that of NaBr. Higher
the strength, greater will be the melting point and higher will
be the dissociation constant.
[next page]
|