|
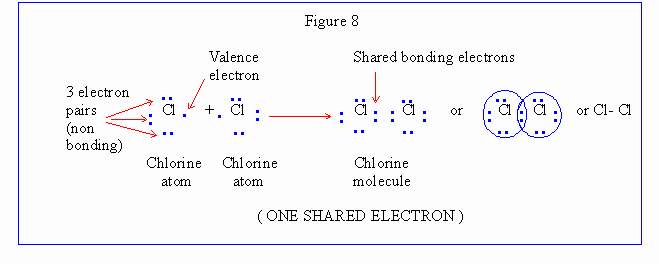
4.3 Covalent Bond
A Covalent Bond is formed due to sharing of electrons between two
atoms.
When both the atoms ( similar or dissimilar ) taking part in a chemical combination are short of electrons to complete the nearest inert gas configuration, the combination between them takes place by sharing electrons. Each atom contributes one electron to form a common pair which then is shared by both.
The Bond established between atoms by this process of sharing is known as a covalent bond.
Chlorine molecule is represented as in Figure 8.
Click here to
enlarge
A covalent linkage is expressed by a dash (-) in writing
formula. This system of representation of formula of a substance
is known as electron dot (lewis) and dash formula.
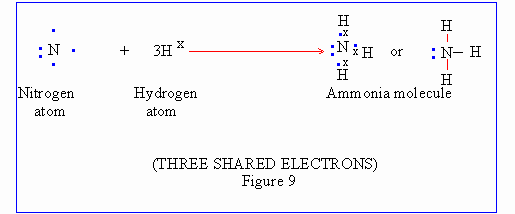
Other examples of covalent compounds where dissimilar atoms combine are, NH3 and CH4. Their formation is illustrated as follows :
Click here to enlarge
[next page]
|