|
4.2 The Ionic Bond
An ionic bond is formed by transfer of electrons from one atom to another atom in the molecule.
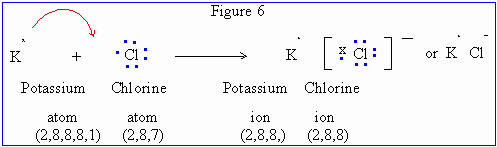
Consider the formation of potassium chloride. An electron is transferred from potassium atom to chlorine atom. The resulting K+ and Cl- ions possessing configuration of argon (2,8,8) combine to form an electrovalent compound as shown below :
Click here to enlarge
Figures in the parenthesis indicate electron distribution in different orbitals
(Electronic configuration) in ground state.
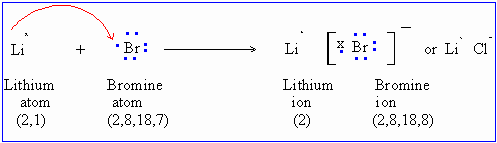
Another example :
 Click here to enlarge
Click here to enlarge
The total number of electrons lost by Lithium must equal the total number of electrons gained by Bromine. Thus the number of Lithium ions produced is the same as the number of bromide ions produced. These ions attract each other to form crystals as shown below.
[next page]
|