|
Limitations of the Octet Rule :
The Octet rule is not adequate as it fails to explain the following points of bond formation.
- Incomplete octet : For atoms of compounds possessing less than eight electrons.
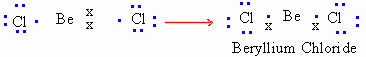
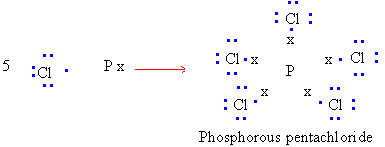
2) Expanded octet : For atoms of compounds possessing
more than eight electrons.
Covalency :
The number of electron pairs which an atom can share with other atoms is known as the covalency of that element.
|
Atoms |
H |
Cl |
O |
N |
C |
P |
|
Covalency |
1 |
1 |
2 |
3 |
4 |
3 |
[next page]
|