|
If 10 dm3 of nitrogen gas originally at 20.26 KPa is
allowed to reach a pressure of 40.52 KPa while keeping temperature
constant, final volume can be calculated as follows :
P1 = 20.26 KPa P2 = 40.52 KPa
V1 = 10 dm3
V2 = ?
According to Boyle's Law
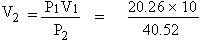
= 5 dm3
It can be seen that since volume and pressure are inversely proportional, on increasing pressure, the volume decreases.
Example :
The volume of a certain gas at constant temperature was found to be 14 liters when the pressure was 1.2 kg/ cm2 If the pressure is decreased by 30% find the final volume of the gas.
V1 = 14 liters P1 = 1.2 kg/cm2
V2 = ? P2 = P1 - 30%
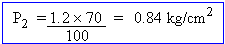
According to Boyle's law
P1V1 = P2V2
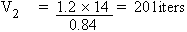
The final volume of the gas is 20 liters.
|