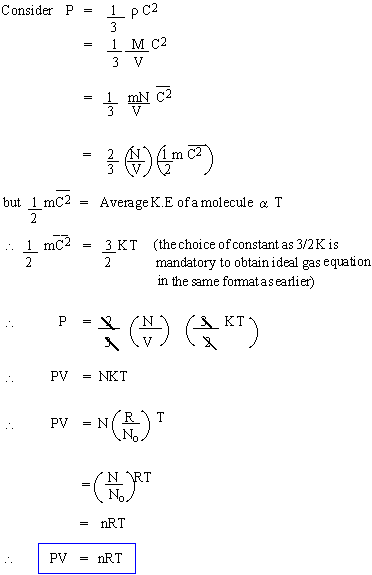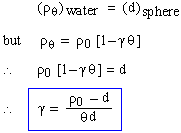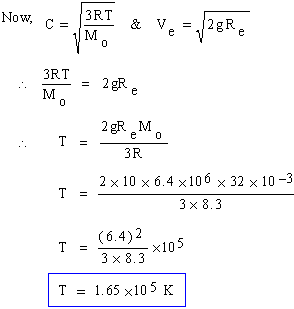|
Ideal gas equation from Kinetic Theory
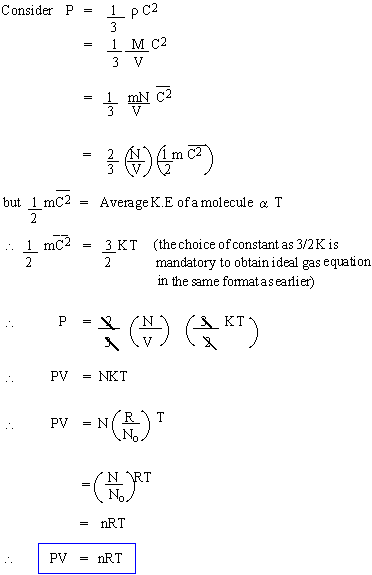
The assumption 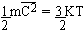 implies the interpretation of heat energy as mechanical energy of
the molecules, albeit as statistical concept only; that is, temperature
is the manifestation of the average motion of a large number of
molecules; it is absurd to say that
implies the interpretation of heat energy as mechanical energy of
the molecules, albeit as statistical concept only; that is, temperature
is the manifestation of the average motion of a large number of
molecules; it is absurd to say that 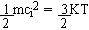 for any i.
for any i.
Solved Problems
-
A hollow metal sphere is floating on water at
00C. If the temperature of water rises to q0C, the
sphere just submerges in water. Neglect the expansion of sphere. Find expression for determining
coefficient of cubical expansion of water.
Solution
Given d : density of sphere,
r : density of water, g : Coefficient of cubical expansion of liquid.
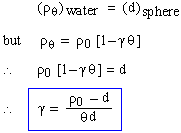
-
Estimate the temperature at which oxygen molecules can escape from Earth. (g = 10 m/s,
Re = 6.4 ´ 106 m, R = 8.3 Joule/mole ´
0K, Molecular wt. of O2 = 32)
Solution :
Given : C = R.M.S. velocity ,
Ve = escape velocity from Earth then the molecules can escape.
If C = Ve then the molecules can escape.
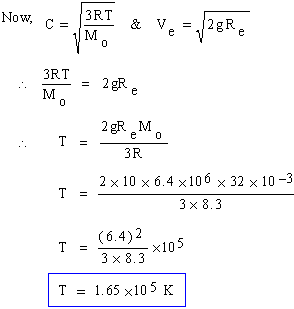
**********
|
Index
12.1 Solids
12.2 Liquids
12.3 Gases
12.4 Gas Laws
12.5 Equation of State :Ideal Gas Equation
12.6 Kinetic Theory of Ideal Gases
Chapter 13
|