|
3.3 Atomic Weights of An Elements
The atomic weight of an element is defined as a number which expresses the ratio of weight of one atom of an element to the weight of one atom of hydrogen.

For e.g. The atomic weight of nitrogen is 14 which
implies that an atom of nitrogen is 14 times heavier than one atom
of hydrogen.
In recent times, the naturally occurring isotope of carbon C12 is taken as standard.
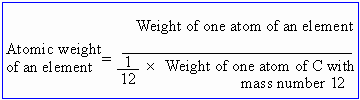
Thus atomic weight is only a number. It has no
units and it does not provide any information regarding the actual
weight of an atom of an element.
Fractional Atomic Weight :
A large number of naturally occurring elements consists of a mixture of isotopes (atoms of the same elements with the same atomic number but different atomic masses). The properties of these isotopes are constant and hence an element has a fixed atomic weight. The atomic weight of an element thus represents the average of atomic masses of different isotopes of the element. This in some cases leads to fractional atomic weight.
For e.g. Chlorine possesses two isotopes with atomic masses 35 & 37 in the proportion of 3 : 1.
Hence,
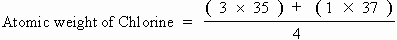
= 35.5
Gram atomic weight :
The atomic weight of an element expressed in grams is known as gram atomic weight or 1 gm. atom of an element.
e.g. 1 gm. atom of Bromine = 79.9 gms. of Bromine
1 gm. atom of Sodium = 22.98 gms of Sodium
At a glance

|