|
Ionic Radius :
The force of attraction or repulsion, F, between two charges qC (charge on cation) and qA (charge on anion) separated by a distance d (sum of ionic radii of two ions) is given by Coulomb’s law as
Where D = dielectric constant.
d = radius of cation + radius of anion as shown in figure.
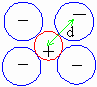
Figure 7 Ionic radii
|
Table 6 Ionic Radii (in 0 A) |
|
CATIONS |
ANIONS |
|
Group I A |
Group II A |
Group III A |
Group VI A |
Group VII A |
|
E |
R |
E |
R |
E |
R |
E |
R |
E |
R |
|
Li+ |
0.60 |
Be2- |
0.31 |
|
|
O2- |
1.4 |
F- |
1.36 |
|
Na- |
0.95 |
Mg2+ |
0.65 |
A13+ |
0.50 |
S2- |
1.84 |
Cl- |
1.81 |
|
K+ |
1.33 |
Ca2+ |
0.99 |
Ga3- |
0.62 |
Se2- |
1.98 |
Br- |
1.95 |
|
Rb+ |
1.48 |
Sr2+ |
1.13 |
In3+ |
0.81 |
Te2- |
2.21 |
r- |
2.16 |
|
Cs+ |
1.69 |
Ba2- |
1.35 |
Ti3+ |
0.95 |
|
|
|
|
[next page]
|
Index
4.1
Introduction
4.2 The Ionic Bonds
4.3 The Covalent Bonds
4.4 The Polar Bonds
4.5 Electronegativity
4.6 Others Bonds
Chapter 5
|