|
Let’s use the Kb and Kf chart and the above
formulae to solve the following problems.
Example
What will be the freezing point and boiling point of a solution containing 3.8 gms of benzoic acid in 50 gms of chloroform ?
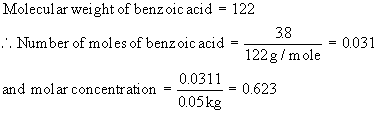
Freezing point depression
D Tf = 0.623 ´ 4.68 =
2.915
So, the freezing point of chloroform solution
- 63.5 - 2.91 = - 66.4150C
Boiling point elevation
D Tb = 0.623 ´ 3.63 =
2.261
So, the boiling point of chloroform solution
61.2 + 2.261 = 63.460C
Example
What will be the molecular weight of the solute if a solution prepared from 500 gms of a solute in 3 kg of water has a boiling point elevation f 20C than that of pure water ?
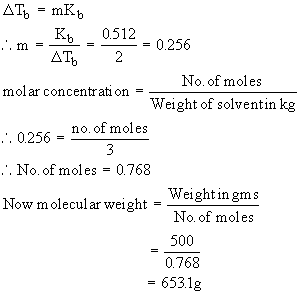
**********
[next chapter]
|