|
9.2 Electrolytic Cell
A device which converts chemical energy to electrical energy is
called electrolytic cell, and the process of conversion is
called electrolysis. e.g. Voltameter used for electrolysis
of water. Electricity is conducted by an electrolyte which is nothing
but a liquid solution present in an electrochemical cell.
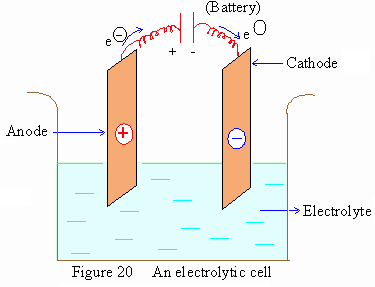
Electrolytic cell : It consists of a vessel with two
electrodes dipped in an electrolyte. The electrode attached to the
negative terminal of an external source of energy (Battery)
is called the cathode and the one which connects to the positive
terminal is called the anode.
|
Index
9.1 Introduction
9.2 Electrolytic Cell
9.3 Electrolytes
9.4 Faraday's Law of Electrolysis
9.5 Electrochemical Cell
9.6 Electrode Potential
Chapter 10
|