|
Like wise pressure of gas on walls ^ r to Y and Z axis are respectively, 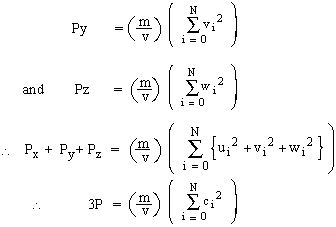
[ For convenience only we have treated Px , Py , Pz are different, or we have determined pressure thrice over as all N molecules were considered to be moving along X, Y and Z all the three axis.] 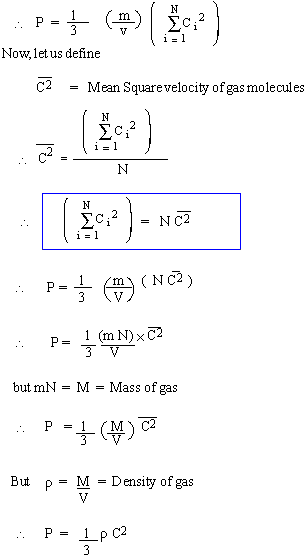
Finally, we define Root Mean Square (R.M.S) velocity C of molecules as Square root of the Mean Square Velocity. 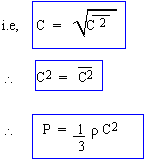
It is customary to call this equation, i.e., pressure expression, as the equation of state. In the above derivation of pressure expression, all but the last assumption viz, Average K.E of any molecules is proportional to the absolute temperature of the gas, has been invoked at some stage or the other. |
12.1 Solids
Follow @Pinkmonkey_com  |
