|
Example
Calculate the pH value of 1) 0.1m HCl 2) solution obtained by mixing 5 gms of conc. HCl to get 1 liter volume.
1) \ pH = -
log10 [
H+ ]
= - log10
[0.1]
= - log10
(1 ´ 10-
1 ) = +1
2) Molar concentration = 5
36.46 g/mole
= 0.137 molar
\ pH = -
log10 [
H+ ]
= - log10
(0.137)
= - log10
(1.37 ´
10- 1 ) = 0.86
pH indicators
An indicator is a chemical which indicates the nature of the solution, by means of a sharp change in color.
|
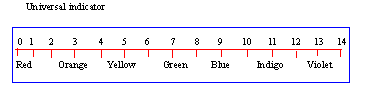
Universal indicator
|
Acid-base indicator
|
- Is a mixture of organic dyes
- Indicates strength of solution
- Change in color is sharp at any pH
|
- is a chemical such as Methyl orange, phenolphthalein
- Does not indicate strength of solution
- change in its color is abrupt at a particular pH
|
[next page]
|